Savings Copay Card
Please complete the form below to receive your patient savings copay card. You may qualify to save on your prescription of SUTAB with this coupon.
Terms and conditions apply, see below for more details.
Savings Copay Card
You may qualify to save on your prescription of SUTAB. Please select an option below to find out more.

*Indicates a required field.
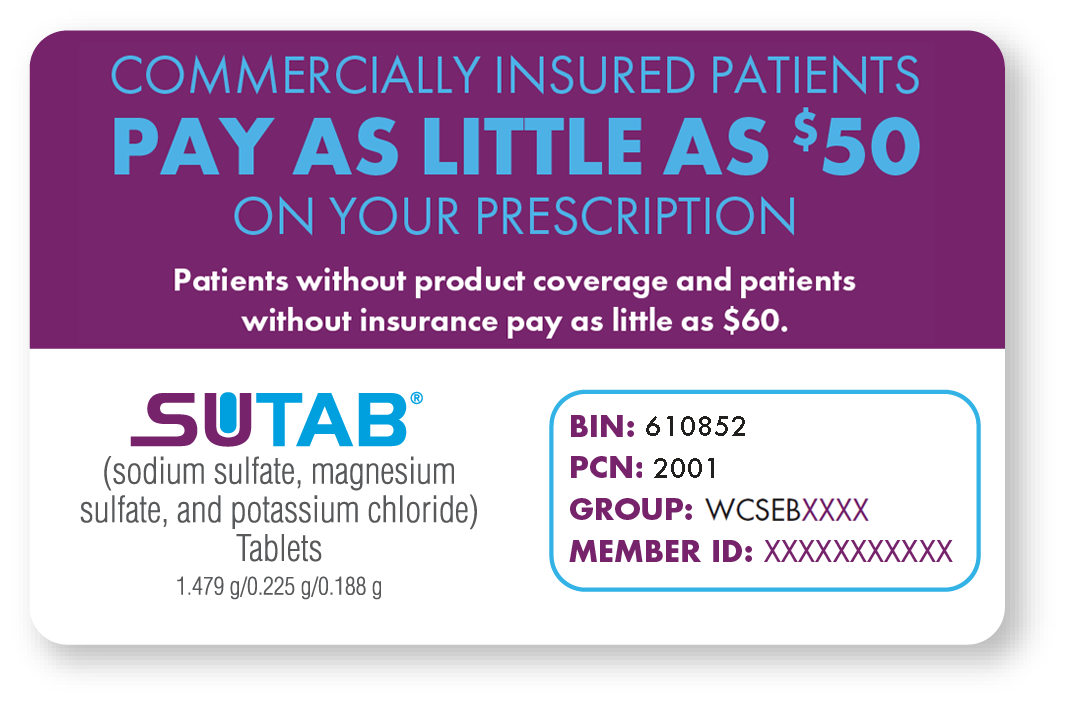
To Patient: Present this card to your pharmacy along with a valid prescription for SUTAB®. Commercially insured patients with product coverage will receive savings up to the program maximum after paying the first $50.00. Patients without product coverage and patients without insurance will receive savings up to the program maximum after paying the first $60.00. Any additional amounts due are your responsibility. This offer is limited to one use and is not transferable. By using this card, you acknowledge that you meet the eligibility criteria and will comply with the terms and conditions. If you have any questions, call 1-844-926-4140.
Pharmacist Instructions for Commercially Insured Patient with Product Coverage: Submit the claim to the primary Third Party Payer first, then submit the balance due to Capital Rx as a Secondary Payer COB with patient responsibility amount and a valid Other Coverage Code (OCC 8). The patient is responsible for the first $50.00 and reimbursement for the balance, up to the program maximum, will be received from Capital Rx.
Pharmacist Instructions for Commercially Insured Patient without Product Coverage: Submit the claim to the primary Third Party Payer first, then submit the balance due to Capital Rx as a Secondary Payer COB with patient responsibility amount and a valid Other Coverage Code (OCC 3). The patient is responsible for the first $60.00 and reimbursement for the balance, up to the program maximum, will be received from Capital Rx.
Pharmacist Instructions for a Cash Paying Patient: Submit this claim to Capital Rx. A valid Other Coverage Code (e.g. 0,1) is required. The patient is responsible for the first $60.00 and reimbursement for the balance, up to the program maximum, will be received from Capital Rx.
For pharmacy processing questions, please call 1-844-306-9173.
Eligibility Criteria: This coupon is not valid for prescriptions reimbursed under Medicare, Medicaid, or any other federal or state program, or where prohibited by law. Offer valid only for prescriptions filled in the United States. Patients must be 18 years or older to participate. Braintree Laboratories, Inc. reserves the right to discontinue this offer at any time. It is a violation of federal law to buy, sell, or counterfeit this certificate. Offer expires December 31, 2024.
INDICATION
SUTAB® (sodium sulfate, magnesium sulfate, potassium chloride) tablets for oral use is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
CONTRAINDICATIONS
Use is contraindicated in the following conditions: gastrointestinal obstruction or ileus, bowel perforation, toxic colitis or toxic megacolon, gastric retention, hypersensitivity to any ingredient in SUTAB.
DOSAGE AND ADMINISTRATION
A low residue breakfast may be consumed. After breakfast, only clear liquids may be consumed until after the colonoscopy. Administration of two doses of SUTAB (24 tablets) are required for a complete preparation for colonoscopy. Twelve (12) tablets are equivalent to one dose. Each SUTAB bottle contains a desiccant. Remove and discard the desiccant from both bottles the evening prior to the colonoscopy. Water must be consumed with each dose of SUTAB and additional water must be consumed after each dose. Complete all SUTAB tablets and required water at least 2 hours before colonoscopy.
WARNINGS AND PRECAUTIONS
Risk of fluid and electrolyte abnormalities: Encourage adequate hydration, assess concurrent medications and consider laboratory assessments prior to and after each use; Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk; Seizures: Use caution in patients with a history of seizures and patients at increased risk of seizures, including medications that lower the seizure threshold; Patients with renal impairment or taking concomitant medications that affect renal function: Use caution, ensure adequate hydration and consider laboratory testing; Colonic mucosal ulcerations: Consider potential for mucosal ulcerations when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease. Suspected GI obstruction or perforation: Rule out the diagnosis before administration. Hypersensitivity reactions, including anaphylaxis: Inform patients to seek immediate medical care if symptoms occur. Risk of Gastrointestinal Complications with Ingestion of Desiccant: Postmarketing reports of ingestion of the desiccant along with SUTAB tablets has been reported and may be associated with risk of gastrointestinal complications and/or choking.
ADVERSE REACTIONS
Most common gastrointestinal adverse reactions are: nausea, abdominal distension, vomiting, and upper abdominal pain.
DRUG INTERACTIONS
Drugs that increase risk of fluid and electrolyte imbalance.
View the Full Prescribing Information and Medication Guide.
References: 1. IQVIA, National Prescription Audit Report. December 2023. 2.Di Palma JA, Bhandari R, Cleveland M, et al. A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 2021;116(2):319-328. doi: 10.14309/ajg.0000000000001020. 3. SUTAB® [package Insert]. Braintree, MA: Braintree Laboratories, Inc. 4. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 (corrected). Am J Gastroenterol. 2009;104(3):739-750.
SUFLAVE™ (polyethylene glycol 3350, sodium sulfate, potassium chloride, magnesium sulfate, and sodium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
DOSAGE AND ADMINISTRATIONA low residue breakfast may be consumed on the day before colonoscopy, followed by clear liquids up to 2 hours prior to colonoscopy. Administration of two doses of SUFLAVE are required for a complete preparation for colonoscopy. Each bottle must be reconstituted with water before ingestion. Each bottle and one flavor enhancing packet are equivalent to one dose. An additional 16 ounces of water must be consumed after each dose. Stop consumption of all fluids at least 2 hours before the colonoscopy.
CONTRAINDICATIONSUse is contraindicated in the following conditions: gastrointestinal obstruction or ileus, bowel perforation, toxic colitis or toxic megacolon, gastric retention, hypersensitivity to any ingredient in SUFLAVE.
WARNINGS AND PRECAUTIONS
Risk of fluid and electrolyte abnormalities: Encourage adequate hydration,
assess concurrent medications and consider laboratory assessments prior to and after each use; Cardiac arrhythmias: Consider pre-dose and
Most common adverse reactions (≥2%) are: nausea, abdominal distension, vomiting, abdominal pain, and headache.
DRUG INTERACTIONSDrugs that increase risk of fluid and electrolyte imbalance.
